Personalizing immunotherapy using bispecific antibodies
Although much of the world was focused on beating the COVID-19 pandemic over the past year, devastating diseases like cancer, Alzheimer disease, and heart disease continued to impact millions of individuals. Fortunately, research and progress towards treatments and cures for these diseases continued with many exciting and groundbreaking discoveries taking place.
It would take us a while to work through all of the cool studies that have come out since the start of the pandemic, so we will start with a more recent one in the field of cancer immunotherapy. A set of studies published in March 2021 in Science and Science Immunology describe a potentially promising new therapeutic for cancers with specific genetic mutations.
But before we get into the nitty-gritty of the study, let’s dive into the world of cancer immunotherapy for a moment.
Teaching immune cells to seek and destroy cancer
While a drug to cure cancer remains a holy grail for scientists and doctors, some of the most promising advancements in cancer treatment recently have come from rallying the body’s own natural defenses. Scientists have made major strides using genetics to summon a more aggressive response from the human immune system. The approach, called immunotherapy, works by teaching the immune system how to recognize cancerous cells and how to attack them.
One type of immunotherapy that has had success and is approved by the FDA for use in patients with acute lymphoblastic leukemia and lymphoma is chimeric antigen receptor (CAR) T cell therapy. For this therapy, doctors extract a patient’s T cells and genetically engineer them to seek out a specific protein on the surface of tumor cells. The cells are reintroduced back into the patient’s body, with the goal of seeking out and killing cancerous cells expressing the specific protein.
Another type of immunotherapy uses tumor infiltrating lymphocytes (TILs) which are immune cells that have naturally gained entry into tumors and are actively engaged in tumor destruction. To begin TIL therapy, scientists analyze the DNA of the patient’s tumor to identify mutations present in the cancer. Then they extract TILs from the tumor, specifically searching for those that will react to the patient’s specific cancer mutations. The chosen TILs are grown in a lab until billions of cells are present, then the cells are infused back into the patient’s body to seek out and eliminate tumor cells expressing the specific mutations.
While these immunotherapies show promise, they are time-consuming and expensive to make because the treatments must be manufactured for each individual. In addition, they sometimes cause nasty side effects. Therefore, scientists are looking for new ways to “teach” immune cells to detect and kill cancer cells without affecting healthy cells.
Immune cells aren’t the only stars of the immune system
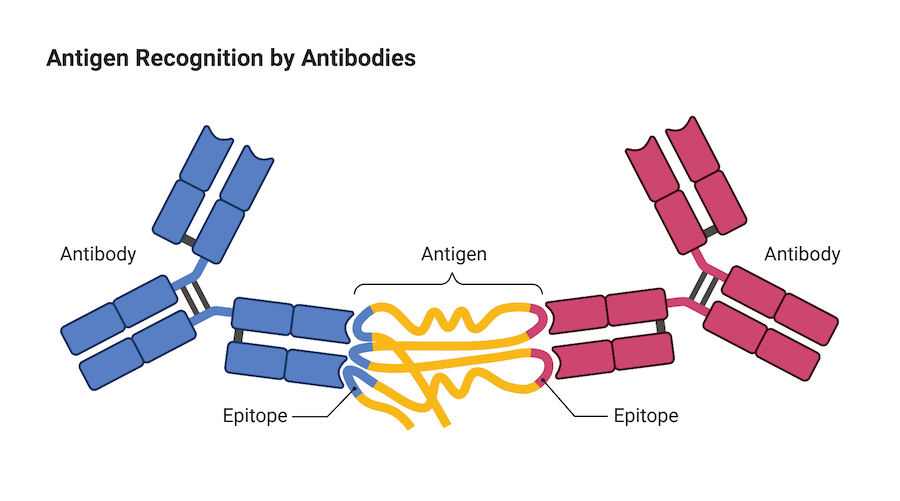
Immune cells are not the only warriors our immune system employs to protect us against invaders like viruses, infection and cancer, antibodies are also important members of the fight. Antibodies are small, Y-shaped proteins produced by a type of white blood cell called a B cell. When they come into contact with a foreign invader, B cells produce antibodies and release them into the blood circulation where they can seek out and neutralize the foreign invader.
The end of each arm of the Y-shaped antibody is like a key that binds onto a protein on the surface of the foreign invader called an antigen. Once an antibody binds to the antigen, it either directly neutralizes the threat itself, or marks the invader so that other cells in the immune system can locate it and remove it from the body.
Because of their ability to bind to harmful pathogens, chemicals, or proteins on a cell’s surface, lab-engineered antibodies have become the basis of many therapeutics over the past decade. For example, the drug adalimumab (brand name Humira), is a monoclonal antibody that treats rheumatoid arthritis by inhibiting an inflammatory protein known as a cytokine.
Creating antibodies that can grab hold of rare antigens
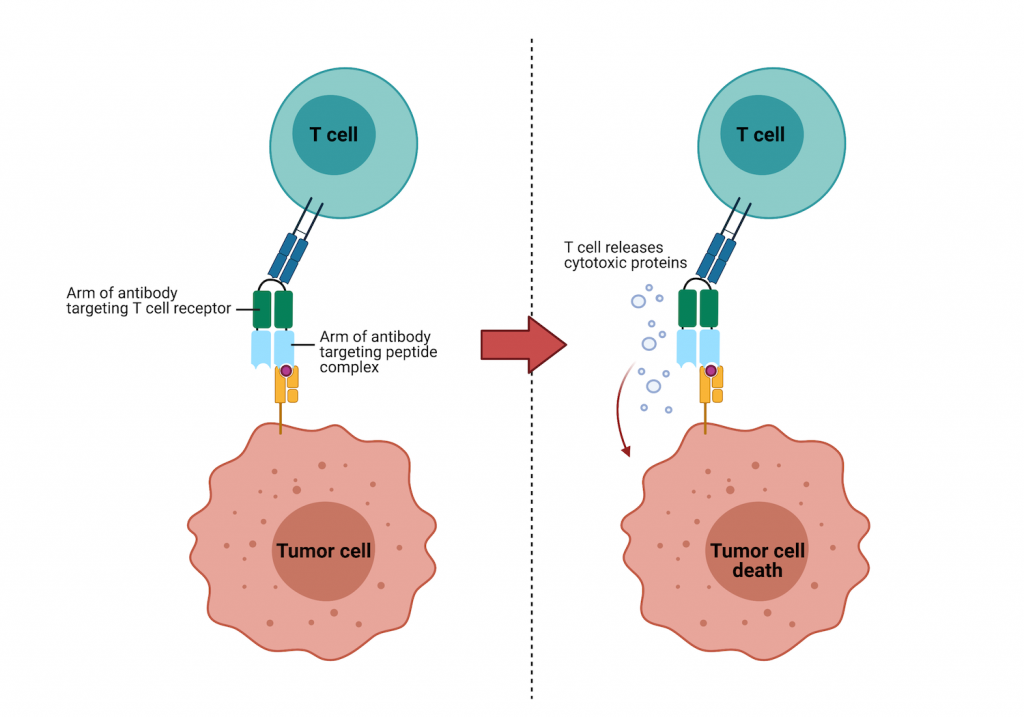
Researchers from the Ludwig Center at Johns Hopkins Medicine engineered bispecific antibodies to bind to cancer cells and recruit immune cells to the cancer cell to destroy it. Instead of binding the same antigen with both arms of the antibody, bispecific antibodies can bind one antigen with one arm and a totally different one with the other arm.
Bispecific antibody technology is not extremely new—the FDA approved the first bispecific antibody for use in patients with acute B cell lymphoblastic leukemia in 2015 and has since approved at least five others. The novel part of these new bispecific antibodies is their ability to identify and bind to cancer cells by targeting mutated antigens that often differ from the normal version by only one amino acid. This would be like a detective solving a robbery because she recognized a tiny stain on the robber’s clothing that matched wet paint at the crime scene.
In healthy cells, p53 protein plays a key role in controlling cell division and cell death. By halting the division of cells with mutated or damaged DNA, p53 helps prevent the development of tumors. Mutations in the gene that encodes p53 allow the cell cycle to proceed unchecked meaning mutated cells can freely grow and spread throughout the body. Although many cancers carry mutations in the gene that encodes p53, there is not a good therapeutic target to the mutant version.
Cancer cells present fragments of the mutant p53 on their surface in a complex with a protein called human leukocyte antigen (HLA) – that’s the tiny paint stain from our earlier analogy. The Johns Hopkins team figured out how to target the rare mutant p53-HLA complex with one arm of their bispecific antibody. The other arm targets a protein on T cells. Once the T cell and the cancer cell are both bound to the antibody, the T cell secretes molecules that destroy the cancer cell.
Using a mouse model of cancer, the research team showed that the bispecific antibody not only stopped tumor growth, it also shrunk the tumors. They showed similar success with a bispecific antibody that targeted proteins made from mutant RAS, another gene commonly mutated in cancer.
The results from these studies are promising and exciting, but they are just the first step in the transition of the therapy from preclinical development to clinical trials. The team still needs to optimize the antibodies and show that they are safe for humans. If the technology is ultimately safe and successful in humans, it could provide a more cost-effective cancer treatment with fewer side effects.