Some of the most advanced technologies in the world are currently being leveraged to fight against mosquitoes. While these backyard pests seem to irritate everywhere they go, they also pose enormous health risks.
Dr. Francis Collins, the Director of the National Institutes of Health, notes in a blog post:
Mosquitoes are the deadliest animals in the world. More than 3.2 billion people—about half of all humans—are at risk for malaria, and more than 400,000 die each year from the disease. Other mosquito-borne illnesses, including Zika and dengue viruses, sicken millions more each year. By combining existing insect control strategies with the latest technical innovation, it should be possible to lower those numbers.
Now scientists say they have promising test results for a new tool to fight the pests—a genetically engineered fungus.
As always when dealing with genetics, the little nuances make all the difference. In order to understand the project, we have to explore the details.
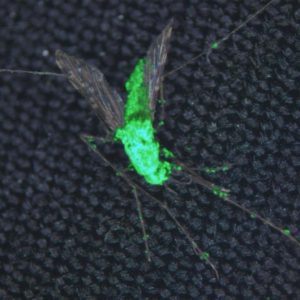
This composite image shows a dead female Anopheles gambiae, killed by Metarhizium pingshaense, which has been engineered to produce spider and scorpion toxins. The fungus is also engineered to express a green fluorescent protein for easy identification of the toxin-producing fungal structures.
Credit: Brian Lovett
Engineering a Fungus
How do you engineer a fungus to beat a bug?
Very carefully.
In this case, researchers started with Metarhizium pingshaense, a fungus that naturally kills off mosquitoes. Problem being that the wild type of the fungus (the typical version found in nature) doesn’t kill the insects off particularly quickly. The mosquitoes still have time to bite their host and pass on disease.
The scientists needed a way to boost the potency of the fungus, so they used genetic engineering to add a toxin from the Australian Blue Mountains funnel-web spider. The gene for this toxin was only activated when the fungus came in contact with the mosquito’s hemolymph, the insect version of blood. Introducing these mechanisms allowed a much smaller number of spores to kill the mosquitoes in a shorter time span.
Safety in Mind
Whenever you get into questions of genetic engineering, safety quickly becomes a concern from a variety of angles. Can you introduce the change without it hurting people? What about the local ecosystems? The list of questions goes on and on.
So what steps do researchers take in regards to ensuring their genetic creation is safe?
For starters, the toxin itself, the one taken from the spider, is actually approved by the Environmental Protection Agency as a safe-and-effective insecticide. Attaching it to a mechanism that only activates when in contact with hemolymph serves as another check on the toxin, by limiting its contact with the environment at large.
 The fungus also offers a number of “safety features.” For example, it does not survive long in sunlight. This makes it harder for the fungus to spread outside of the structure interiors where it is applied.
The fungus also offers a number of “safety features.” For example, it does not survive long in sunlight. This makes it harder for the fungus to spread outside of the structure interiors where it is applied.
Then there’s the actual testing process. The research team had already shown the modified fungi rapidly killed mosquitoes in a laboratory setting, but needed to test their hypothesis in a setting that more closely mimicked real-world conditions. So, they created a “MosquitoSphere” to contain the experiment. A large dome of insect nets was erected in Burkina Faso, containing several compartments that mimic the huts and conditions of a rural village. The researchers put the modified fungus in some of the huts, the unmodified fungus in others and no fungus in the remaining set. In the huts with the modified fungus, mosquito populations were cut by 75% in fourteen days and essentially eliminated within 45 days.
Even still, it’s important to note that the technology is nowhere near deployment and remains in the early testing phase.
One Weapon in an Arsenal
It’s tempting to hope researchers will find a silver bullet in circumstances like this, but it’s important to view tools like this one as a single prong of a multipronged approach to a problem.
 One of the main reasons global eradication efforts for malaria-carrying mosquitoes have stalled is that the mosquitoes have become resistant to the insecticides most typically used.
One of the main reasons global eradication efforts for malaria-carrying mosquitoes have stalled is that the mosquitoes have become resistant to the insecticides most typically used.
Late last year, we wrote about an effort to eliminate mosquito populations using a mechanism called a gene drive, where you introduce mutations into a population that inhibit breeding. It’s important to note we’re not looking at a race to find the one solution to mosquitoes spreading malaria. We’re racing to see how many effective arrows we can put in the quiver.
This is, in part, because not every solution will work in every situation. However, there’s a broader vulnerability to a singular approach. If researchers focused all their efforts on, say, introducing a fungus that killed mosquitoes, then what happens if the insects evolve a resistance?
These tools can take decades to develop. Each one has the potential to make an important impact, but we need to make sure we don’t treat any of them as a cure-all.
Balancing Act
In many ways, this effort to control malaria-carrying mosquito populations reflects the most pressing questions in genetics. We can see the near-endless potential of genetic engineering; there is a real, human impact on the table right in front of us. Reducing these mosquito populations would save lives.
Still, carefully studying the ripple effects in the genome of the organisms involved, in the environment and in the communities where the solution would be deployed cannot be rushed. We see these same balances come into play with genetically engineering crops to withstand changing climates and developing genetic treatments for disease.
Genetics offers hope for the future, but often comes with weighty decisions for today.
To schedule a media interview with Dr. Neil Lamb or to invite him to speak at an event or conference, please contact Margetta Thomas by email at mthomas@hudsonalpha.org or by phone: Office (256) 327-0425 | Cell (256) 937-8210
Get the Latest Sharable Science Delivered Straight to Your Inbox!
[gravityform id=19 title=false description=false ajax=true][wprpw_display_layout id=8]